Conventional medical professionals look to clinical trials and other research to safely and effectively recommend pharmaceuticals to their patients. Integrative medical practitioners must do the same prior to recommending supplements or other nutritional interventions, especially when recommended in conjunction with pharmaceutical prescriptions. In either case, a responsible practitioner should refer to the existing body of evidence to make informed clinical decisions in order to develop the safest and most effective treatment plan. However, most practitioners, whether conventional or integrative, simply do not have the time to read and critically appraise the accumulating body of evidence for dietary supplement interventions prior to developing integrative treatment plans for patients. For this reason, Evidence-based medicine and supplement ingredient research will give you better insight into the recommendation and use of dietary supplements.
Evidence-based medicine
Evidence-based medicine (EBM) is a combination of three key principles. It involves locating and applying relevant scientific evidence, integrating patients’ values and preferences in therapy, and using clinical and expert judgment to optimize patient health outcomes. EBM prioritizes evaluating the body of research on a topic instead of simply following established norms or standards.
Critical appraisal of research
Research is not created equally. Even within the context of peer-reviewed literature, research is subject to confounding variables, bias, and issues of reliability, or internal and external validity. F Our Health Coaching Team systematically conducts literature searches related to specific ingredients and conditions, as well as drug-nutrient depletions and interactions, and synthesizes this information as educational content under the bounds of an established rating scale. The rating scale at the end of this document defines, prioritizes, and represents the research used in our educational content. With it, you can easily understand the context through which recommendations (eg. protocols, dosing, timing, etc.) have been made for dietary supplements.
A-level of evidence
Our Health Coaching Team prioritizes systematic reviews and meta-analyses of human trials, which are combinations of findings from multiple studies to form a consensus on a clinical hypothesis. They are often considered the highest level of evidence available, providing a rank of A-level evidence in the our Rating Scale.
B-level of evidence
Randomized, double-blind placebo-controlled (RDBPC) trials are also rated relatively highly as B-level evidence but should consist of at least 50 subjects. While a sample size of n = 30 is generally considered necessary for statistical significance, a sample size of 50 participants is favorable for RDBPC studies based on our team’s research experience and margin of error tolerance. RDBPCs are considered the gold standard in conducting clinical trials.
C-level of evidence
Randomized controlled trials (RCT), which may lack methodological procedures to control bias (e.g., no placebo, no blinding) whether by the fault of the study design itself or the need to adhere to ethical standards, are rated moderately as represented by C-level evidence. These trials can provide moderate-level evidence via comparison to a specified control group. RDBPCs with less than 50 subjects are also represented under this level of evidence to account for population sizes.
D-level of evidence
Human trials with no comparison group, lacking controlled study designs, or retrospective analyses are ranked alongside animal trials with D-level evidence. These human trials often solely examine the effect of a single intervention without the inclusion of a relevant comparable intervention or are limited to measuring associated outcomes. Animal trials are a pre-clinical means to examine potential physiological relationships between an intervention and an outcome but are not necessarily generalizable to the human population.
E- and F-level of evidence
In vitro and other studies that purely examine mechanistic relationships between an ingredient and human physiology are ranked with E- and F-levels of evidence, respectively. While these studies may highlight properties of an ingredient particularly at the molecular level, they cannot be solely used to make conclusions on the effects of an ingredient in human health. They are nonetheless included to provide a perspective that the preliminary research has been conducted.
Evidence-based medicine: an evolving process
In an era where accessing unlimited information from an infinite number of sources is possible, clinicians must be able to critically appraise the information that is presented to them. This is of utmost importance for ensuring both safe and effective treatment of all patients.
However, the true critical appraisal of research also necessitates analyzing the dogma of EBM, which conventionally relies on populationist philosophies. These studies are designed to treat the majority of a specific population under a refined set of physiological and environmental circumstances, using a specific therapeutic protocol. Inherently, these research methods present tremendous value in the demonstration of the (lack of) effectiveness of an intervention, but can also present issues of generalizability. For example, a therapy that works for one individual may not work for another.
Thus, in addition to using EBM in clinical practice, integrative practitioners should understand that the art of personalized care may require transcendence from the sole reliance on EBM to the establishment of a practitioner-patient relationship that permits the discovery of alternative modes of treatment that supports the patient as a whole.
Ultimately, these considerations necessitate the critical appraisal of research in peer-reviewed journals, but it also invites the opportunity to evolve research dissemination as a whole.
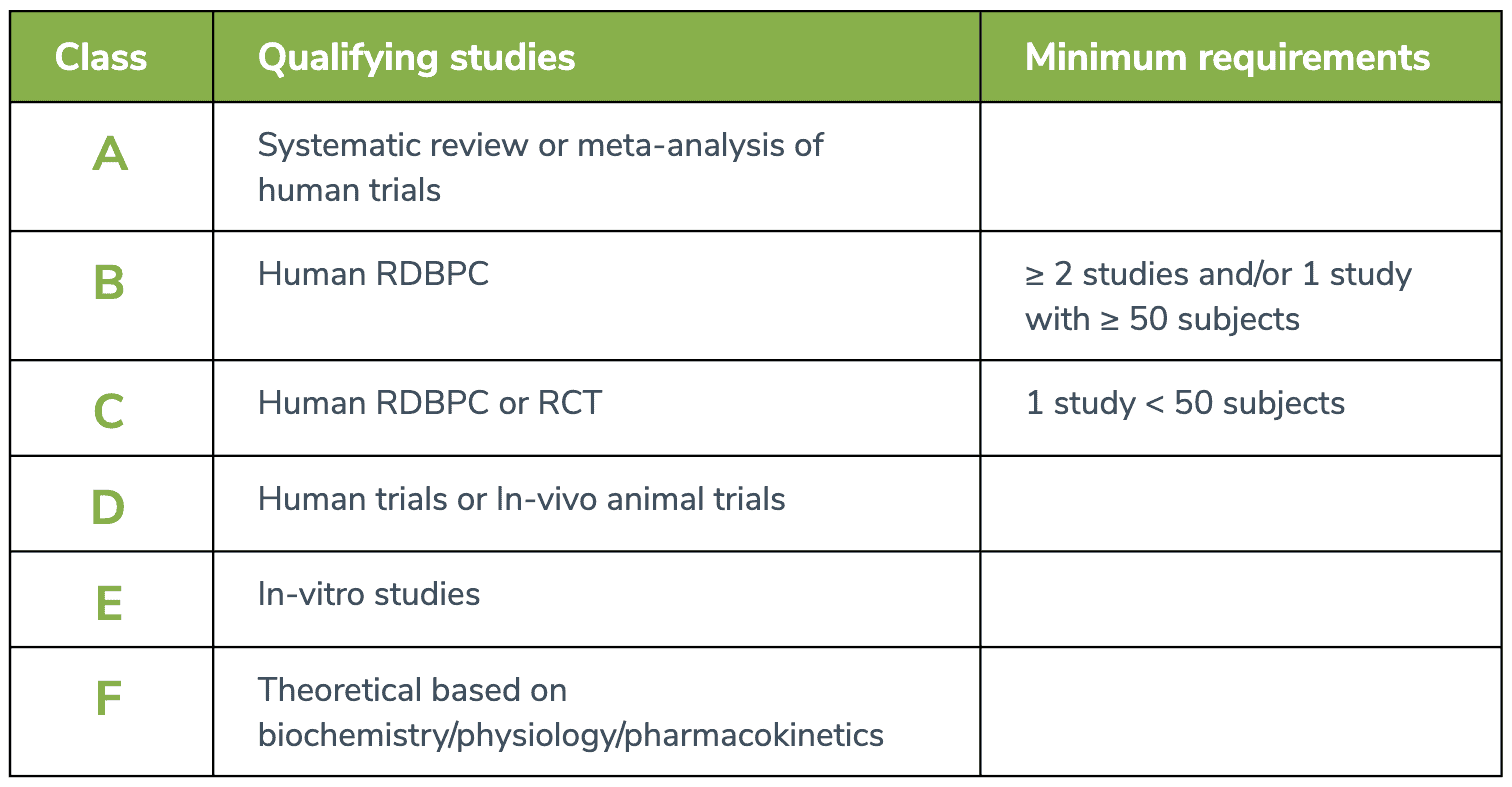 The rating scale at the end of this document defines, prioritizes and represents the research used in our educational content.
The rating scale at the end of this document defines, prioritizes and represents the research used in our educational content.
RDBPC = Randomized Double-Blind Placebo-Controlled RCT = Randomized Controlled Trials

